Salem Clemens talks to John Sorkness about Carpet Anemones, how to ID them, keep them, propagate them, and maybe even spawn them!
Tell us what got you into the hobby, how long you’ve been in it, and your passion for gigs.
I was first introduced to aquariums at the age of 9 when my parents gave me a small acrylic aquarium with guppies and neon tetras. That aquarium multiplied into half a dozen freshwater aquariums scattered through my bedroom and bathroom in my youth. 20 years ago I converted one of my freshwater tanks into my first saltwater tank and have not stopped ever since.
As long as I can remember fish and coral have always captivated me. I got my first anemone with that initial saltwater aquarium, a condylactis anemone, and was forever hooked on anemones. As I learned from many mistakes at that age and gained more experience, my attention turned to Entacmaea quadricolor (common BTA) until I discovered Heteractis magnifica. Once I saw my first H. magnifica anemone I was really obsessed with anemones. It wasn’t until 2018 that I got my first Stichodactyla gigantea, a tiny bleached baby blue gigantea, that my obsession pivoted to the gigantea anemone.
The gigantea has several qualities that have captured my devotion: the density of tentacles that are long enough to hide a clownfish, but that don’t get so ridiculously long that they sweep into other corals, the bright vibrant colors that are hard to find in other species, the way the disc of the anemone folds into gentle rolling contours, and the fact that once the anemone is happy, it secures it’s spot and never ventures around the aquarium.

What phenotypes of gigs do you currently have, and are there any on your wish list?
I currently have blue, purple, and the ever-coveted red. As silly as it sounds, I wish I had a neon green. Neon green was one of my first giganteas and it, unfortunately, got shaded out by a blue to the point it withered away to nothing. I would love to get that color back in my tank.
Could you break down the different kinds of “carpet anemones” for those new to them, including an explanation of any anatomical features such as siphonoglyphs, etc?
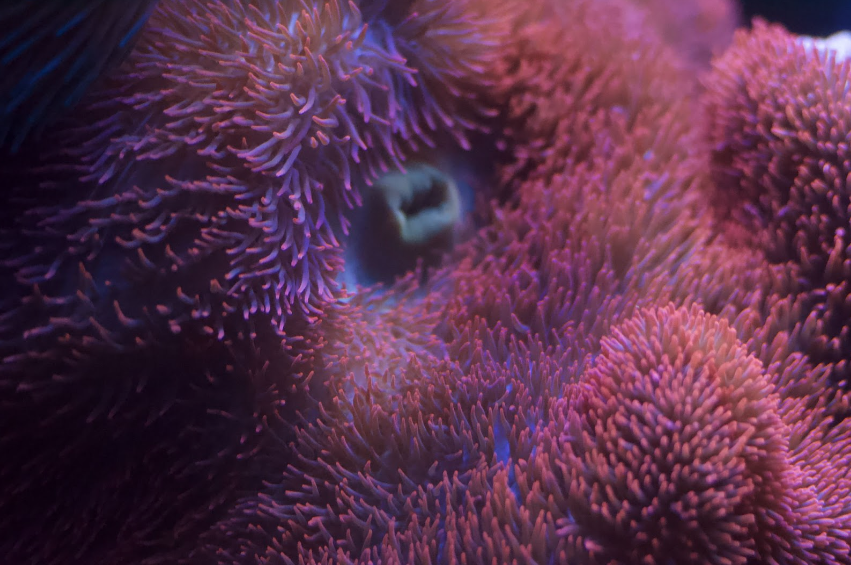
There are several species of anemones that are commonly known as “carpet anemones”. Some of them host clownfish while others don’t. The most common clownfish hosting anemones are S. haddoni, S. mertensii, and S. gigantea. Mertensii anemones grow very large, have verrucae (colored dots found cascading the column of the anemone). They are typically found in earthy colors with dense mats of short tentacles.
Haddoni anemones can be found in a wide range of colors, but typically live in the sand and have dense, very short and stubby tentacles. Their columns are light colored and they don’t have the verrucae down the sides of the column that S. gigantea have.
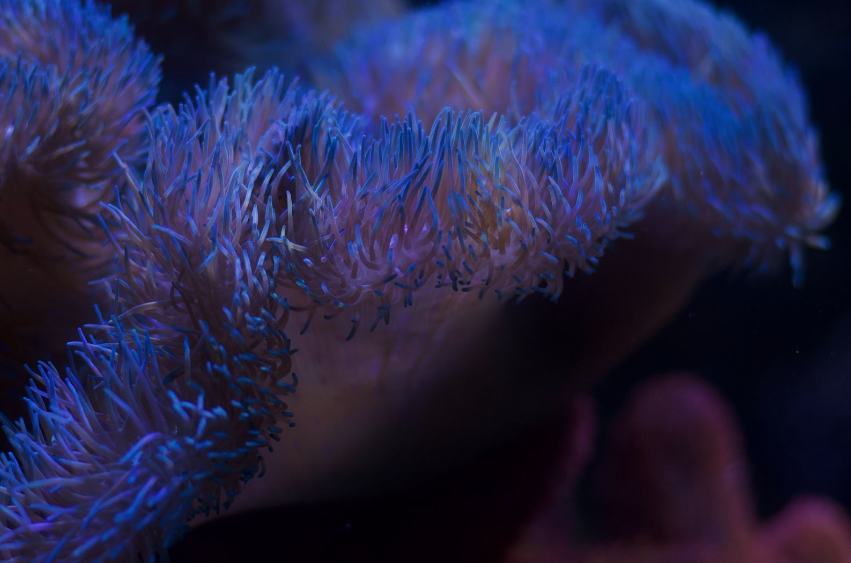
S. gigantea anemones can be found in a wide range of colors, mainly blue, purple, tan, green, and a mixture of colors. They have blue columns with purple verrucae cascading down the sides of the column. Most of these larger anemones have what is known as siphonoglyphs at the entrance of the mouth. It is believed they are associated with inflating and deflating the anemone. S. gigantea typically have a set of two on opposite sides of the mouth from each other. Other common species known as “carpet anemones” that don’t host clownfish include C. adhaesivum (pizza anemone), S. tapetum (maxi mini anemone), and S. helianthus (the Atlantic carpet anemone that is commonly mistaken as a haddoni).

Are there easy ways to tell the difference between the different carpet anemones – is genetics sometimes required?
Yes, within the hobby it is commonly agreed that haddoni don’t have verrucae or blue columns, their tentacles are typically short and bulbous, and can be seen lining the pedal of the anemone in roughly organized rows. Gigantea anemones have blue columns with bright purple verrucae down the columns. Their tentacles are usually longer with the tops of the tentacles being colored and the base of the tentacles usually a beige earthy color. Genetic testing could be helpful for confirming, but is a costly test that is not readily available to most hobbyists.

You explore propagating gigs by cutting them on your Reef 2 Reef thread. Have you had long-term success with this method, and if so, could you break down the protocol?
I have had great success and I continue to do so. A few years ago it was widely agreed that gigantea anemones could not be manually propagated through cutting since they are so delicate and would die from the split. I experimented with a protocol of cutting the anemone that has so far worked. My protocol is as follows:
· Sterilize a sharp pair of scissors with rubbing alcohol
· Cut the anemone straight through the mouth leaving a siphonoglyph on either side. Be sure to perfectly cut the anemone through the middle including the base of the foot and the pedal disc of the anemone
· Place the two halves in a hospital tank with an air stone and a HOB filter with a foam cover to provide gentle flow. Treat the water nightly with ciprofloxacin for a week. I typically treat a 5-gallon tank with 250 mg of ciprofloxacin nightly.
· After the anemones have finished 7-10 days of treatment, move them into a basket in the aquarium until they heal fully. They do take a long time to recover and for the mouth to move back to the center of the anemone and reform a second siphonoglyph.
I have several purple and blue gigantea that I have split once. I plan on splitting those splits again soon once I feel confident they are large enough and have the second siphonoglyph in the mouth. I hope to keep splitting these anemones long term so we can reduce our collection in the wild by providing maricultured gigantea in the hobby. The largest barrier is the time it takes to propagate these cuts.
What are some tips for keeping gigs happy? PAR? Spectrum? Flow? Feeding?
High light, stable parameters, and strong and variable flow. As far as spectrum goes they seem pretty happy around 300-400P PAR. Once they are happy they do not move. They will situate themselves on the side or tops of rock structures. I have my powerheads nearby to give them strong and variable flow. Their long tentacles like to be pushed around a lot and giving them a direct strong flow will create a corkscrew-shaped tentacle. I don’t typically feed the gigantea in my display tank as feeding them regularly will make them grow very large. I do feed chunks of shrimp to my propagated giganteas every other week or so to encourage them to grow faster. I have started feeding my red gigantea more to see if I can get it to spawn in captivity.
A change of topic, but on your thread, you stated you work for the University of Texas at Austin and assist them with ex-situ spawning. Care to break down your role at the university, the goals of the team you are on, and your achievements.
Yes! I was lucky enough to get involved with a coral genetics lab at UT Austin. I was brought on staff part-time in 2019 and have been working with them ever since. Initially, they wanted someone with experience keeping Acropora alive and they asked me to take on that responsibility after the PI visited my home in 2017 and was impressed with the dense and healthy SPS tank I had at the time. The goal shifted to spawning Acropora ex-situ here in Austin so the lab could perform various genetic tests without having to fly to Australia with lab equipment.
I have successfully spawned various species of Acropora and Euphyllia in my home tank over the past few years and continue to do so. While we have had some successful fertilizations that led to useful research data, I have yet to successfully raise a new generation of Acropora. It appears getting Acropora to spawn is the easy part, getting the recruits to survive is the real challenge. My tank does have many babies of Euphyllia scattered throughout the rocks, but they are much easier to care for and spawn than Acropora. I hope to spawn gigantea ex-situ, but so far I have not observed any spawning activity and looking for a successful protocol to do so.
Do you have any tips for successfully spawning corals or published methodology you based your efforts on?
Oh yes, Jamie Craggs has helped pioneer this method and is incredibly successful. His paper is publicly available here: https://onlinelibrary.wiley.com/doi/full/10.1002/ece3.3538
Getting corals to spawn is as simple as programming the diurnal cycles into an Apex. Adjusting the sunrise/sunset, monthly temperature, and lunar cycle is all it takes really. I usually feed the corals heavily in the months leading up to spawning, but I don’t think it’s as necessary as the sharp increase in temperature.
In your thread, you mentioned one instance where you had strange ORP readings that may have been due to a gig spawning event. Since then, have you observed any potential spawning behavior or found literature analyzing how spawning may occur?
It has been a repeating occurrence that ORP begins trending downwards in the nights leading up to a synchronized coral spawn. The release of all that organic material and lipids causes the ORP to dip sharply at the time of spawning. If I am not watching the tank at night when the actual spawning occurs it is impossible to identify who was responsible though.
With Acropora it’s easier to know it was them because they release egg bundles that float to the top. If the tank is just hazy, there’s a chance a broadcast spawner like an anemone or clam was responsible and it is just sperm in the water. There have been those instances in my tank, but again it could be a number of animals that were responsible. I believe gigantea might be brooders, so it could start with a release of sperm in the water that then is taken up by a female and fertilizes internal eggs. The larvae would develop in the mother and then be released to complete their maturity in the ocean. This is just my hypothesis and I hope to observe it from manipulating the seasonal settings of my Apex and feeding the anemones more.
What is your next goal with gigs? Will you focus on physical or sexual propagation? Something else entirely?
Right now I am physically propagating a set of purple and blue gigantea. I would like to keep splitting them manually to get to a point where I could distribute them continuously within the hobby. Compounding growth is slow though when it takes nearly a year to go from 1 to 2 and then 2 to 4. Sexual propagation is my end goal. I know how to trigger Acropora and Euphyllia spawning, but I have not confirmed that environmental shifts alone trigger gigantea spawning, or what those triggers could be. I hope I can figure this out and begin producing sexually propagated gigantea into the hobby. Especially with my red gigantea which I am still too nervous to even consider physically propagating at this time because if it fails, there goes the only known red gigantea in the hobby.
If you would like to read John’s Reef 2 Reef thread find it here:
https://www.reef2reef.com/threads/gig-%E2%80%98ems-grand-gigantea-gathering.850187/



